- Visibility 43 Views
- Downloads 14 Downloads
- DOI 10.18231/j.jsas.2022.008
-
CrossMark
- Citation
Gastrointestinal parasites of surgical importance and their management — A review
Introduction
A parasite (Greek: parasitos) is defined as an “organism that lives in a close association with another organism of a different species (host), derives nutrition from it and is pathogenic to it, although this potential may or may not be always expressed”.[1] Although any infectious agent is, in strict terms, a parasite, medical terminology uses this word in a narrower sense to mean eukaryotic pathogens, the protozoa, and helminthic organisms. Though the connection between humans and parasites seems to have existed since prehistoric times, it was only in the nineteenth century that the discoveries resulting from exploration and colonization were pieced together,[2] Many life cycles of parasites were elucidated and the role of parasites in human disease became evident. Parasitology thus gained a firm footing in medicine. This article reviews a few parasites, which infect the gastrointestinal tract and are of relevance to the surgeon. Important clinical features, laboratory tests, imaging findings, and surgical aspects of management are highlighted.
Entamoeba Histolytica (Amebiasis)
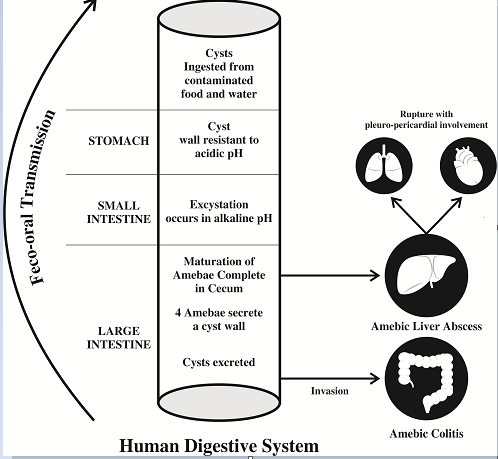
Entamoeba histolytica causes invasive amebiasis. It ranks third in causing death due to parasite disease, after Schistosomiasis and Malaria. This parasite is endemic in areas, where overcrowding, poor sanitation, and low socioeconomic conditions are prevalent.[3] In developed countries, travelers, immigrant populations, homosexual men, HIV-infected individuals, individuals in mental institutions, and prisons constitute high-risk groups. This disease spreads via the fecal-oral route. Humans get infected when they consume food and water contaminated with feces containing amebae.
Pathogenesis of Entamoeba Histolytica
The life cycle of this parasite is depicted in [Figure 1]. In the colon, trophozoites of E.histolytica adhere to the Gal/GalNAc adherence lectin on colonic epithelial cells.[4] This stimulates the secretion of interleukin-8(IL-8) and tumor necrosis factor (TNFα) leading to polymorphonuclear cell infiltration.[5], [6] The resulting interaction leads to inflammatory cell death and necrosis of tissue, exposing the extracellular matrix. The amebae being highly pathogenic, release multiple enzymes like cysteine protease, collagenase, and several polypeptides that degrade host defense molecules such as IgA and complement proteins. This results in widespread destruction of submucosal tissue and the formation of flask-shaped ulcers in the colon (histo = tissue, lytica = death). In some cases, trophozoites gain access to a submucosal venule, and move through the portal circulation, to reach the liver. The right lobe of the liver receives venous blood from the right side of the colon, making it the most common site of abscess formation. The sluggish blood flow in the sinusoids creates a perfect environment for their lodgment and multiplication. Amebae damage the neutrophils, and the lysosomal enzymes so released, which promote hepatocyte death. This produces a cavity filled with blood and liquefied liver tissue, known as “Anchovy sauce pus,” at aspiration.
Clinical Features of Amebiasis
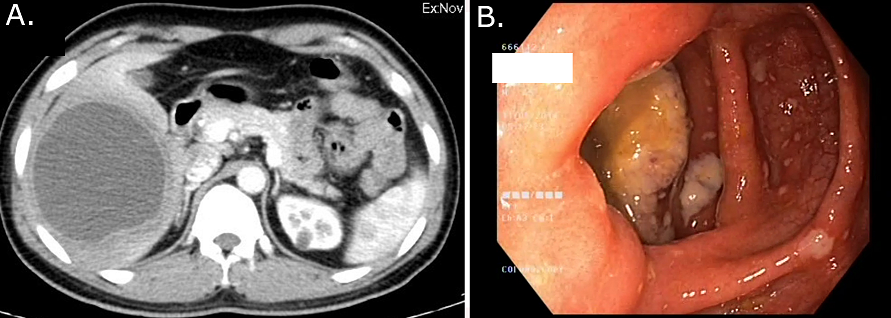
After about 2-6 weeks of the incubation period, the patient presents with lower abdominal discomfort and loose stools.[7] The stool frequency increases in severe disease. The content of the stool is mainly blood and mucus with very little fecal matter. Erosion of a blood vessel in the ulcer bed results in bleeding. Perforation of the ulcer leads to peritonitis. The cecum and rectosigmoid junction are favored sites for the formation of the 'amoebic granuloma' or 'ameboma' ([Figure 2]A), a lesion resulting from a florid inflammatory process surrounding a necrotic area, where the hypertrophic granulation tissue and fibrosis cause the formation of a pseudotumor. This occurs rarely, in about 1-2% of patients with repeated bouts of infection and noncompliance with the treatment. These mass lesions may be mistaken for malignancy and resected, only to reveal the benign nature of the disease.[8]
In the amebic liver abscess, there is sudden onset right-sided abdominal pain. Fever, accompanied by rigors and profuse sweating, may be present. The pain is constant, severe, and increases with deep inspiration or hiccough. The classic sign on physical examination is painful hepatomegaly with guarding in the right upper quadrant. Mild jaundice is common.
Diagnosis of Amebiasis
Blood tests
The laboratory tests may be normal in mild cases of colitis, with leukocytosis occurring in severe disease. The white blood cell count is raised above 10 × 109/L in most patients with amebic liver abscesses. Alkaline phosphatase (ALP) level rises in long-standing disease,[7] Serum levels of transaminases are elevated in 50% of cases and signify acute disease or complications.
Stool microscopy
The classical finding on stool microscopy is the presence of hematophagous trophozoites (i.e., trophozoites which have ingested red blood cells). Microscopy has fallen out of favor as it requires skill and experience on the part of the microscopist and it has a low sensitivity (10-60%).
Serology
Antigen detection using enzyme-linked immunosorbent assay (ELISA) is the preferred investigation in the endemic areas. They show 55% to 100% sensitivity, and 93% to 100% specificity in comparison to the other established testing methods. The antibody detection in serum is better in non-endemic areas. Again, ELISAs are the most sensitive with very low false-negative results. The serological tests should be repeated in 5-7 days, as they may be initially negative in the acute phase of infection.[9]
Radiology
In the acutely ill patient, if a plain erect abdominal radiograph shows colonic dilatation or gas under the diaphragm, surgery must be considered (Toxic megacolon or hollow viscus perforation needs exploration regardless of the etiology). On ultrasound, amebic liver abscesses are often located near the liver capsule presenting as a unilocular, round-oval, hypoechoic lesion with low-level internal echoes and no discernible wall echoes. An adjoining disruption of the diaphragm is highly suggestive of the diagnosis.[10] On CECT, they appear as peripherally enhancing, well-circumscribed, rounded lesions with fluid attenuation of 10-25HU suggesting complex fluid. The thick peripheral wall (3-15mm) may show a ‘target’ or ‘double rim’ appearance with a surrounding rim of edema ([Figure 2]B). The central cavity could show fluid-debris levels or multiple septae too.[11] On MRI, amebic abscesses show homogenous low signal on T1 and high signal on T2 weighted images respectively, simulating the other pyogenic abscesses with perilesional edema in about 50% of cases.
Management of Amebiasis
The amebic infections are treated with two classes of drugs. The luminal amebicides (such as diloxanide furoate and iodoquinol) are poorly absorbed from the intestine, hence acting best on luminal amebae. Tissue amebicides, such as metronidazole, tinidazole, dehydroemetine, and chloroquine reach a high concentration in blood and tissue after oral or parenteral administration. Hence, they are best used in the treatment of amebic abscesses. They are less effective against luminal organisms.
For the liver abscess, combined treatment with metronidazole or tinidazole, followed by diloxanide furoate is recommended. More than 90% respond to this treatment, becoming afebrile in about 3–5 days. Dehydroemetine and emetine can cause nausea, vomiting, and cardiac arrhythmias and are best used as second-line agents.
Role of Surgery in Amebiasis
Routine aspiration or ultrasound/CT guided drainage is avoided, except[12] when the diagnosis is in doubt — serology is inconclusive & pyogenic liver abscess cannot be ruled out; when the antiamebic drugs are contraindicated (e.g. pregnancy); when the secondary infection of the abscess occurs (15% of cases);[13] when fever and pain do not respond to 5 or more days of anti-amebic therapy and when rupture of the abscess into the thorax or pericardial cavity is imminent. The surgical intervention is indicated only for complications.[14]
Colitis with hollow viscus perforation
Laparotomy followed by a thorough peritoneal lavage is done. The decision regarding primary closure of the perforation, resection of the affected segment of bowel, or proximal diversion is made on a case-to-case basis. Postoperatively metronidazole is added to the antibiotic regimen.
Abscess rupture into thoracic cavity
Intercostal tube drainage is tried first, with the positioning of the drain as high as possible on the right lateral side of the chest. Aggressive procedures such as thoracotomy and drainage or decortications may be required in chronic cases or loculated collections.
Abscess rupture into peritoneum
Thorough lavage of the peritoneal cavity is given, and the liver is handled as gently as possible. The temptation to break all “loculi” should be resisted — these loculi are blood vessels and ducts in the abscess cavity. Endoscopic stenting is a feasible option for postoperative bile leaks in case they occur.
Ascaris Lumbricoides (Ascariasis)
The roundworm Ascaris lumbricoides causes Ascariasis. It is the most common human parasitic infection in east and south Asia and sub-Saharan Africa. In endemic areas, about a quarter of the population may be infected.[15]
Life cycle of ascaris lumbricoides and pathogenesis ([Figure 3])
The Ascaris lumbricoides larvae cannot move through the pulmonary capillaries. Hence, they burrow through the alveoli moving retrogradely to the trachea. A strong eosinophilic inflammatory response occurs, causing ‘Charcot Leyden crystals’, along with segments of dead larvae to appear in the sputum.
Adult worms migrate when they sense a disturbance in the environment. Stressful situations like fever, diarrhea, anesthesia, etc. are well-known triggers for worm migration.[16] The migration can occur into the common bile duct (most common) or the gallbladder or the intrahepatic ducts.[17] Sphincterotomy, sphincteroplasty, or bilio-enteric anastomosis reduces the resistance, making migration easier. In addition, the hormone progesterone has a relaxing effect on the sphincter, thereby explaining increased symptoms during pregnancy. Impacted worms cause spasms of the sphincter of Oddi, resulting in obstructive jaundice, suppurative cholangitis, and acute pancreatitis.[18] The worm sometimes dies and disintegrates, eliciting chronic inflammation that leads to the formation of strictures in bile ducts. Fragments of the dead worm or ova can also act as a nidus for the stone formation. This has been demonstrated by some authors on microscopic examination of stones.[19]
Clinical Features of Ascariasis
Light infections do not usually cause symptoms. Acute manifestations occur with an increasing worm burden i.e., more than 100 worms in the GI tract. A pneumonitis occurs 4–16 days after the infection, as the larva migrates through the lungs. Fever, nonproductive cough, and dyspnea may be present (Löffler’s syndrome).[20] Moderate to severe breathlessness, sometimes even leading to status asthmaticus has been reported. The pneumonitis resolves in about 3 weeks. Children below the age of 10 years are prone to develop intestinal obstruction. This is easily understood as the small bowel is narrow in caliber and gets blocked easily due to the high worm burden.[21] Acute onset abdominal distension, vomiting, and constipation occur, and the child becomes sick rapidly. Masses of worms sometimes cause volvulus or intussusception, resulting in bowel gangrene and perforation.
Worms in the biliary tract cause right upper quadrant pain with tenderness and guarding. Jaundice is usually absent. If the worm returns to the duodenum, symptoms subside. However, dead worms and eggs block the duct — leading to stone formation and cholangitis with pain, jaundice, fever with chills, and leukocytosis.[22] Acute pancreatitis may be caused by a worm blocking the pancreatic duct but severe pancreatitis with progression to necrosis is uncommon.[19]
Diagnosis of Ascariasis
Passage of worms in the stool or a history of vomiting the worms usually gives the diagnosis. Stool microscopy shows the ova or worm remnants in the feces. Eosinophilia >5% is rare and if it occurs, the association with Toxocara or Strongyloides spp. infection must be suspected.
Radiological Features of Ascariasis
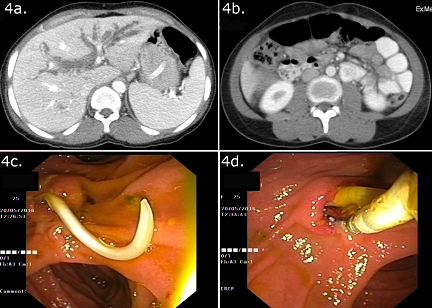
On ultrasound, adult worms are seen in the GB or CBD as non-shadowing tubular echogenic structures with an anechoic central longitudinal alimentary tract of the worm giving a ‘tube within a tube or inner tube’ appearance. Occasionally they present only as curved echogenic linear strips without shadowing (strip sign). Multiple worm infestation may form a bag or cluster in the GB, while in the CBD, the overlapping worm interfaces cause a spaghetti-like appearance. In long-standing infestations, calcified linear structures may be seen suggesting dead worms. The CBD and pancreatic duct dilatation, pneumobilia, and occasionally intrahepatic abscesses may be associated.[23], [24]
On CT ([Figure 4]A and 4B), depending on their orientation within the biliary tree or intestine, worms are seen as round or elongated filling defects.[25] On T2W MRI and MRCP, the worm appears as a hypointense filling defect within the hyperintense bile and is a valuable noninvasive method of detecting ascariasis if ultrasound is equivocal.[26]

Management of Ascariasis
Albendazole and mebendazole are the drugs of choice.[27] Their cure rate approaches 100%. Administering these drugs with a fatty meal enhances bioavailability. Levamisole and pyrantel pamoate can be used as second-line agents.
Role of Surgery in Ascariasis
Mohta et al.[28] elegantly described the management of intestinal obstruction due to ascariasis. Conservative management with analgesics, decompression via nasogastric tube, intravenous fluids can be tried in subacute cases. This is usually successful in most patients. Once the intestinal function has recovered, anthelmintic therapy is initiated. Failure of obstruction to resolve after 48 h or deterioration in the patients' condition mandates surgery.
At laparotomy, the small bowel is milked gently to displace the bolus of worms caudally into the colon; if unsuccessful, enterotomy is done and the worms are manually removed. The enterotomy is best closed using nonabsorbable sutures to prevent penetration by the remaining ascarids. Resection and primary anastomosis can be safely performed in a stable patient with bowel gangrene or perforation, whereas resection and ileostomy is an appropriate strategy in a patient with a poor general condition. All the family members of patients admitted with Ascaris-induced obstruction are treated with anthelmintics. This is a sound strategy in an endemic area against reinfection.
Spontaneous return of the worm occurs in more than 90% of cases of biliary ascariasis.[28] This is beneficial for the patient and may save the need for an interventional procedure. therefore, a trial of conservative treatment with nasogastric aspiration, antispasmodic drugs, and intravenous fluids is instituted. This is discontinued when pain and tenderness settle, and a repeat ultrasound exam shows no worm in the bile duct. If conservative treatment fails, ERCP ([Figure 4]C and 4D) is attempted.[29] The sphincter is often found to be dilated due to the passage of worms, facilitating easy cannulation. Reddy et al.[30] prefer grasping forceps or dormia basket to polypectomy snares as these enable extraction of worms in toto. Complete extraction is important because remnants of worms left behind can lead to calculi formation or obstruction. Anthelmintic therapy paralyzes or kills the worm and interferes with its spontaneous return. The dead worms can fragment when handled, necessitating repeat procedures to clear the duct. Anthelmintic therapy is, therefore, given after the extraction.
Surgery is required for worms in the intrahepatic ducts, with stone and stricture formation. Worms are removed through a longitudinal incision on the CBD. Cholangioscopy is helpful and can ensure the completeness of the procedure. This is followed by saline irrigation and the incision is closed over a T-tube. A T-tube cholangiogram done on day 6 or 7 can identify the residual worms. A worm stuck in the T-tube may be removed by application of the suction to the tube, while withdrawing it. Residual worms can be removed by endoscopy. If these methods fail, repeat surgery may be required.[14], [30]
Clonorchis sinensis, opisthorchis viverrini & opisthorchis felineus
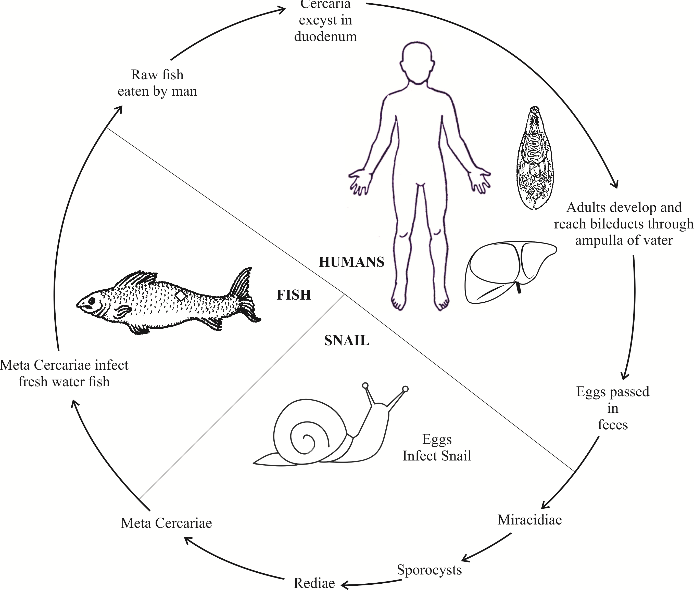
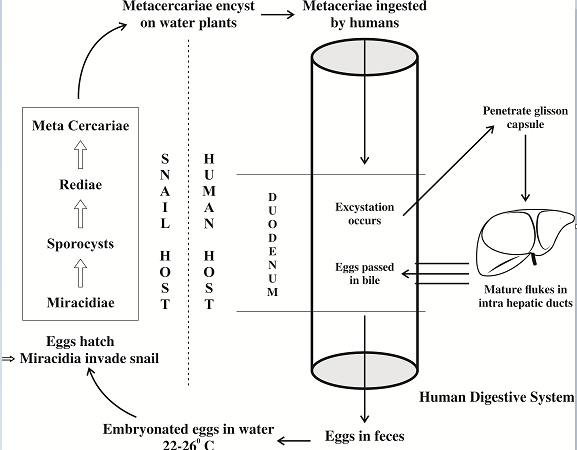
The World Health Organization (WHO) has classified them as biological carcinogens.[31] These parasites are responsible for conditions ranging from cholecystitis, choledocholithiasis, recurrent pyogenic cholangitis to bile duct carcinoma. They are a major public health problem in Southeast Asia and Eastern Europe.[32] Interestingly, the incidence of cholangiocarcinoma is very high in these regions (e.g. Khon Kaen province in Thailand and Qidong in China).[33], [34], [35] These areas have certain traditional eating practices, where people consume raw/fermented fish. The uncooked/fermented fish contain encysted metacercaria, which causes infection in humans. The life cycle of cercariasis is detailed in figure 5.
Pathogenesis of cercariasis
Multiple mechanisms play a part in the pathogenesis of fluke-mediated liver injury.[36] The mechanical factor is that the suckers during feeding can cause biliary ulceration. The metabolic and excretory substances released from tegument and excretory openings are released into the bile. These are highly antigenic as well as toxic to the ductal epithelial cells. There are marked inflammatory infiltration have been observed around the intrahepatic bile ducts in pathologic specimens in animals experimentally infected with Opisthorchis. E.g. O. viverrini Granulin-like substance (Ov-GRN-1) is mitogenic to fibroblast and biliary cell lines in vitro.[37], [38], [39] The liver fluke infection triggers several inflammatory cells (macrophages, mast cells, ductal epithelial cells) to produce various cytokines. One such is nitric oxide (NO) synthesized through the inducible pathway(iNOS).[38] NO reacts with free radicals forming peroxynitrite(ONOO-), which in turn binds to DNA forming 8-OXOdG and 8-nitroguanine adducts inducing DNA damage. [39] Secondly, the antigen of O. viverrini induces NF-kb expression through the TLR2 pathway. NF-kb translocates to the nucleus and turns on several genes, leading to increased production of iNOS and COX-2. COX-2 has an excitatory effect on inflammatory cells with the production of more cytokines. Chronic inflammation with sustained DNA damage may trigger errors in DNA repair and Microsatellite Instability(MSI) setting the stage for carcinogenesis.[40]
Clinical features of cercariasis
Patients present with right upper quadrant pain, fever, rashes, and loss of appetite. O. felineus is commonly associated with arthralgias and lymphadenopathy. Stones in the gallbladder, extrahepatic and intrahepatic ducts can cause cholecystitis, obstructive jaundice, and life-threatening cholangitis.[41], [42] In an endemic area, a patient presenting with cholangitis should be evaluated for cholangiocarcinoma.[43]
Diagnosis of cercariasis
Microscopy of stool, bile, or duodenal aspirates can readily demonstrate the operculated eggs of Opisthorchis or Clonorchis. Any of these methods — The Kato thick smear, Stoll’s dilution, or the quantitative formalin ethyl acetate concentration technique, can be used in moderate and heavy infections.[44]
Serology
The antigen detection in feces by ELISA technique is more sensitive than routine stool examination when parasite load is low(< 500 eggs per gram of stool).[45], [46] Antibody detection techniques typically have low specificity, but the Ov-CP-1–based ELISA with a specificity of 96%, shows some promise.[47] These tests do not distinguish between active and past infection, as antibodies can be detected up to a few months following curative treatment.
Polymerase Chain Reaction (PCR)
The PCR-based tests can be done on fluke DNA in feces, particularly in light infections. The Loop-mediated Isothermal Amplification assay (LAMP) technique is particularly helpful as it allows visual detection of DNA amplification by turbidity, eliminating the need for the thermocycler.[48], [49]
Radiological features of cercariasis
On ultrasound, flukes in the GB may be seen as floating or dependent non-shadowing discrete echogenic foci. In the liver, the flukes cause diffuse dilatation of the peripheral small intrahepatic ducts with characteristically no or very minimal dilatation of large /central ducts and no obvious obstructing lesion.[50], [51], [52], [53] The CT and MRI show similar findings as ultrasound with bile duct thickening and periductal enhancement. In very heavy infestations, MRCP may demonstrate the very thin flukes as low-intensity intraluminal filling defects.[50], [51], [52]
Management of cercariasis
Praziquantel is the drug of choice and has a 71% cure rate. Rim et al.[37] report an 83-85% cure rate in Clonorchiasis when a dose of 25mg/kg is given thrice a day 5 hours apart. There may be mild side effects, such as giddiness, emesis, and abdominal pain. Inflammatory changes and debris within the gallbladder also resolve with the elimination of the parasite. For biliary lithiasis and cholangitis, the choice of procedure depends on the location of stones and/or stricture. Endoscopic approaches are tried first, with surgery reserved for failed cases. Common bile duct exploration and stone retrieval with or without T–tube insertion or hepaticojejunostomy may be done on an individual basis, followed by antiparasitic therapy. Cholangiocarcinoma is managed according to the standard oncologic guidelines.
Fasciola hepatica & fasciola gigantica (Fascioliasis)
These two large liver flukes cause fascioliasis, a chronic inflammatory disease of the liver and biliary system. This disease covers Europe, Africa, the Americas, and Oceania.[53] These flukes primarily infect sheep and cattle, causing considerable mortality. Humans accidentally become final hosts when they eat plants (alfalfa or watercress) or drink impure water contaminated with feces from animals harboring Fasciola.
The life cycle and pathogenesis of fascioliasis ([Figure 6])
After excystation in the duodenum, the larvae migrate through the bowel wall transmurally into the peritoneal cavity. They penetrate the Glisson’s capsule and burrow through the substance of the liver, to reach the bile ducts, where maturation takes place. Symptoms occur during this phase. A strong eosinophilic response results in parenchymal cell death, parasite death, and hemorrhage. Repair mechanisms lead to extensive nodule formation which is often mistaken for malignancy. In chronic infection, mature flukes feed on the hepatocytes and ductal cells and live for many years in the biliary system. They release eggs into the bile that is excreted in feces. Mechanical obstruction of the ducts, secretion of proline by the fluke, and its spinous processes damaging the biliary epithelium all contribute to causing bile duct proliferation, dilatation, and fibrosis.[54]
Clinical features of fascioliasis
Acute infection is the result of the movement of the larva through the liver and its accompanying hemorrhage. Patients present with fever, constitutional symptoms, itching, urticaria, night sweats, and upper abdominal pain. This occurs may occur 12 -16 weeks after the ingestion of metacercaria. Anemia, hepatomegaly, and splenomegaly may be present. Of note, blood investigations show leukocytosis with eosinophilia in almost all patients.
Chronic infection is usually asymptomatic. Mild jaundice may be present. Serum levels of Alkaline phosphatase and Gamma-glutamyl transpeptidase are raised indicating cholestasis. Ulcers in the bile duct due to adult flukes can cause haemobilia. Choledocholithiasis, cholangitis, pancreatitis, biliary cirrhosis, and hepatic fibrosis have all been reported.[55]
Diagnosis of fascioliasis
Fascioliasis is usually diagnosed by observation of the eggs during microscopy of stool, bile, and duodenal contents. Egg detection has often been unsatisfactory. This may be because they cannot produce eggs (Fasciola larva may or may not attain maturity in the human tissues as humans are not their natural hosts) or the eggs get encapsulated in granulomas. This results in infection with low egg shedding and necessitates sedimentation techniques to increase egg detection. Also, differentiation of eggs between various Fasciola species can be difficult.[56]
Serology
Immunodiagnosis seems to be superior to microscopy — the antigen and the antibody can be detected much before egg shedding begins.[57] The more purified the antigen, the better is the antibody detection rate. The cathepsin CL1(a cysteine protease) and the Fasciola 27kDa antigen have been used in several studies, with the latter showing excellent results (98.2% sensitivity and 100% specificity)[58] The antigen detection uses monoclonal or polyclonal antibodies against specific antigens (Circulating Antigen, Excretory Secretory antigen, or ES) in urine or stool samples using a sandwich ELISA. The study by Demerdash et al. for detection of ES antigen resulted in a diagnostic efficacy of 94% and 97% in serum and stool samples respectively.[59] These tests do not differentiate between various Fasciola species and do not quantitatively measure the parasite burden.[60]
Radiological features of fascioliasis
Hepatic fascioliasis may present on ultrasound as multiple confluent, ill-defined hypoechoic nodules which are usually subcapsular. Minimal gallbladder wall thickening and dilatation of intrahepatic biliary radicles are often seen due to associated cholangitis. If seen, the parasite may resemble curvilinear or leaf-like echoes ranging from 5-25mm.[61] On CECT, the ill-defined subcapsular nodules are seen to follow the portal triads and converge towards the hilum of the liver with hypodense linear inflammatory tracts seen in the subcapsular regions. MRI may confirm the segmental geographic areas of hepatic inflammation, subcapsular-periportal hypointense tracts, and micro abscesses with rim enhancement which on diffusion-weighted images may show restricted diffusion. Post-inflammatory fibrosis may be seen as hypointensity on T1 and T2 weighted images.
Management of fascioliasis
A single dose of Triclabendazole 10 mg/kg eliminates both immature and adult parasites with 90% cure rates and is recommended as the treatment of choice. Bithionol has been used as an alternative, with good results.[62], [63]
Role of surgery in fascioliasis
Most patients present or are referred to the surgeon with cholecystitis or obstructive jaundice and choledocholithiasis. Alban et al, even in an endemic area, have recorded only a 1.2% incidence of adult worm in the gall bladder in their series of 162 cholecystectomies.[64] The anti-parasitic medications may kill the flukes and this, in turn, may obstruct the ampulla, causing biliary obstruction and cholangitis. Urgent Endoscopic retrograde Cholangiopancreatography (ERCP) is necessary to remove the dead worms and ensure good drainage of CBD. If ERCP fails, Percutaneous transhepatic cholangiography (PTC) may be necessary. When the pancreatic duct is involved, Parsak et al. recommend drainage followed by Triclabendazole with an antispasmodic to prevent sphincter of Oddi spasm.[65] It was reported that life-threatening pancreatitis seems to be uncommon.[66]
Conclusion
Given the increasing incidence of international travel, parasitic diseases have assumed an even greater significance. These diseases may present a confusing picture to the unwary physician in a non-endemic area, leading to a departure from the optimal line of management, which in turn leads to increased health care costs and patient dissatisfaction. The physician’s suspicion of a parasitic disease may be aroused only if he possesses a good working knowledge of these conditions. Early diagnosis and treatment at the individual level with health education and awareness at the community level plays a vital role in the control of parasitic diseases.
Source of Funding
None.
Conflict of Interest
None.
References
- F H Kayser, K A Bienz, J Eckert, R M Zinkernagel. Medical Microbiology.. Med Microbio 2005. [Google Scholar]
- F E G Cox. History of human parasitic diseases. Infect Dis Clin North Am 2004. [Google Scholar] [Crossref]
- V Gathiram, T F H Jackson. A Longitudinal Study of Asymptomatic Carriers of Pathogenic Zymodemes of Entamoeba Histolytica. South Afr Med J 1987. [Google Scholar]
- S A Adams, S C Robson, R E Kirsch, V Gathiram, T F H G Jackson, T S Pillay. Immunological Similarity between the 170 KD Amoebic Adherence Glycoprotein and Human Β2 Integrins. Lancet 1993. [Google Scholar] [Crossref]
- K B Seydel, E Li, P E Swanson, S L Stanley. Human Intestinal Epithelial Cells Produce Proinflammatory Cytokines in Response to Infection in a SCID Mouse-Human Intestinal Xenograft Model of Amebiasis. Infect Immun 1997. [Google Scholar] [Crossref]
- Y Yu, K Chadee. Entamoeba Histolytica Stimulates Interleukin 8 from Human Colonic Epithelial Cells without Parasite-Enterocyte Contact. Gastroenterology 1997. [Google Scholar] [Crossref]
- E B Adams, I N Macleod. Invasive amebiasis. I. Amebic dysentery and its complications. Med (Baltimore) 1977. [Google Scholar] [Crossref]
- K Saha, M Sengupta, S Mitra, S Ray. Amoeboma of Colon Mimicking Colonic Carcinoma. Tropical Parasitology. 2014. [Google Scholar]
- R Fotedar, D Stark, N Beebe, D Marriott, J Ellis, J Harkness. Laboratory Diagnostic Techniques for Entamoeba Species. Clin Microbiol Rev 2007. [Google Scholar] [Crossref]
- M J Landay, H Setiawan, G Hirsch, E E Christensen, M R Conrad, Thoracic Hepatic, Amaebiasis. Hepatic and thoracic amaebiasis. AJR Am J Roentgenol 1980. [Google Scholar] [Crossref]
- G Elizondo, R Weissleder, D D Stark, L E Todd, C Compton, J Wittenberg. Amebic Liver Abscess: Diagnosis and Treatment Evaluation with MR Imaging. Radiology 1987. [Google Scholar] [Crossref]
- P W Ralls, M F Quinn, W D J Boswell, P M Colletti, D R Radin, J Halls. Patterns of Resolution in Successfully Treated Hepatic Amebic Abscess: Sonographic Evaluation. Radiology 1983. [Google Scholar] [Crossref]
- V G Mcdermott. What Is the Role of Percutaneous Drainage for Treatment of Amebic Abscesses of the Liver?. AJR Am J Roentgenol 1995. [Google Scholar]
- H Dabbous, H S Amiri, G Zibari. Amebiasis and Other Parasitic Infections. Blumgart’s Surg Liver 2012. [Google Scholar]
- S P Misra, M Dwivedi. Clinical Features and Management of Biliary Ascariasis in a Non-Endemic Area. Postgrad.Med.J 2000. [Google Scholar]
- P K Mishra, A Agrawal, M Joshi, B Sanghvi, H Shah, S V Parelkar. Intestinal Obstruction in Children Due to Ascariasis: A Tertiary Health Centre Experience. Afr J Paediatr Surg 2008. [Google Scholar] [Crossref]
- T Kamiya, T Morishita, R Reredo, C Marancenbaum, C Montaño. Duodenoscopic Management in Biliary Ascariasis. Dig Endosc 1993. [Google Scholar] [Crossref]
- N A Wani, O J Shah, S H Naqash. Postoperative Biliary Ascariasis: Presentation and Management - Experience. World J Surg 2000. [Google Scholar] [Crossref]
- M S Khuroo, S A Zargar, R Mahajan. Hepatobiliary and Pancreatic Ascariasis in India. Lancet 1990. [Google Scholar] [Crossref]
- W Löffler. Transient Lung Infiltrations with Blood Eosinophilia.. Int Arch Allergy Immunol 1956. [Google Scholar]
- De Silva, N R Guyatt, H L Bundy, D A. Morbidity and Mortality Due to Ascaris-Induced Intestinal Obstruction. Trans R Soc Trop Med Hyg 1997. [Google Scholar] [Crossref]
- O J Shah, S A Zargar, I Robbani. Biliary Ascariasis: A Review. World J Surg 2006. [Google Scholar]
- N P Ferreyra, G G Cerri. Ascariasis of the Alimentary Tract, Liver, Pancreas and Biliary System: Its Diagnosis by Ultrasonography. Hepatogastroenterology 1998. [Google Scholar]
- M S Khuroo, S A Zargar, R Mahajan, R L Bhat, G Javid. Sonographic Appearances in Biliary Ascariasis. Gastroenterology 1987. [Google Scholar] [Crossref]
- M De S Rocha, N S S Costa, J C G Costa, M T De C Lessa Ângelo, J R Lessa Ângelo, L Sonoda. CT Identification of Ascaris in the Biliary Tract. Abdom Imaging 1995. [Google Scholar]
- C Das, J Kumar, J Debnath, A Chaudhry. Imaging of Ascariasis. Australas Radiol 2008. [Google Scholar] [Crossref]
- A Bennett, H Guyatt. Reducing Intestinal Nematode Infection: Efficacy of Albendazole and Mebendazole. Parasitology Today 2000. [Google Scholar]
- C.-C Chang, C.-T Han. Biliary Ascariasis in Childhood. A Clinical Analysis of 788 Cases. Chin Med J 1966. [Google Scholar]
- D N Reddy, P V J Sriram, G V Rao. Endoscopic Diagnosis and Management of Tropical Parasitic Infestations. Gastrointest Endosc Clin N Am 2003. [Google Scholar] [Crossref]
- N A Wani, R K Chrungoo. Biliary Ascariasis: Surgical Aspects.. World J Surg 1992. [Google Scholar]
- . . Health and Safety Authority, U. S. Biological Agents 2015. [Google Scholar]
- J Keiser, J Utzinger. Food-Borne Trematodiases. Clin Microbiol Rev 2009. [Google Scholar]
- B Sripa, S Kaewkes, P Sithithaworn, E Mairiang, T Laha, M Smout. Liver Fluke Induces Cholangiocarcinoma. PLoS Med 2007. [Google Scholar] [Crossref]
- B Sripa, C Pairojkul. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 2008. [Google Scholar]
- H R Shin, J K Oh, E Masuyer, M P Curado, V Bouvard, Y Fang. Comparison of Incidence of Intrahepatic and Extrahepatic Cholangiocarcinoma - Focus on East and South-Eastern Asia. Asian Pacific J Cancer Prev 2010. [Google Scholar]
- H.-J Rim. Clonorchiasis: An Update. J Helminthol 2005. [Google Scholar] [Crossref]
- M J Smout, T Laha, J Mulvenna, B Sripa, S Suttiprapa, A Jones. A Granulin-like Growth Factor Secreted by the Carcinogenic Liver Fluke, Opisthorchis Viverrini, Promotes Proliferation of Host Cells. PLoS Pathog 2009. [Google Scholar] [Crossref]
- P Yongvanit, S Pinlaor, H Bartsch, Oxidative, Dna Nitrative, Damage. Key Events in Opisthorchiasis-Induced Carcinogenesis. Parasitol Int 10.1016/j.parint.2011.06.011. [Google Scholar]
- S Inoue, S Kawanishi. Oxidative DNA Damage Induced by Simultaneous Generation of Nitric Oxide and Superoxide. FEBS Lett 1995. [Google Scholar] [Crossref]
- S Pinlaor, S T Oikawa, Y Hiraku, P Pinlaor, N Ma, P Sithithaworn. Opisthorchis Viverrini Antigen Induces the Expression of Toll-like Receptor 2 in Macrophage RAW Cell Line. Int J Parasitol 2005. [Google Scholar]
- S Pungpak, M Riganti, D Bunnag, T Harinasuta. Clinical Features in Severe Opisthorchiasis Viverrini. Southeast Asian J Trop Med Public Health 1985. [Google Scholar]
- H J Rim. The Current Pathobiology and Chemotherapy of Clonorchiasis. Korean Jo Parasitol 1986. [Google Scholar]
- S Sayasone, O Rasphone, M Vanmany, P Vounatsou, J Utzinger, M Tanner. Severe Morbidity Due to Opisthorchis Viverrini and Schistosoma Mekongi Infection in Lao People’s Democratic Republic. Clin Infect Dis 2012. [Google Scholar] [Crossref]
- P Sithithaworn, S Tesana, V Pipitgool, S Kaewkes, C Pairojkul, B Sripa. Relationship between Faecal Egg Count and Worm Burden of Opisthorchis Viverrini in Human Autopsy Cases. Parasitology 1991. [Google Scholar] [Crossref]
- W Chaicumpa, Y Ruangkunaporn, T Kalambaheti, S Limavongpranee, V Kitikoon, S Khusmith. Specific Monoclonal Antibodies to Opisthorchis Viverrini. Int J Parasitol 1991. [Google Scholar] [Crossref]
- S Sirisinha, R Chawengkirttikul, M R H Elkins, D B Elkins, S Kaewkes, P Sithithaworn. Evaluation of a Monoclonal Antibody-Based Enzyme Linked Immunosorbent Assay for the Diagnosis of Opisthorchis Viverrini Infection in an Endemic Area. Am J Trop Med Hyg 1995. [Google Scholar] [Crossref]
- D Watthanakulpanich, J Waikagul, M T Anantaphruti, P Dekumyoy. Evaluation of Bithynia Funiculata Snail Antigens by Elisa-Serodiagnosis of Human Opisthorchiasis. Southeast Asian J Trop Med Public Health 1997. [Google Scholar]
- X Q Cai, M J Xu, Y H Wang, D Y Qiu, G X Liu, A Lin. Sensitive and Rapid Detection of Clonorchis Sinensis Infection in Fish by Loop-Mediated Isothermal Amplification (LAMP). Parasitol Res 2010. [Google Scholar] [Crossref]
- Y Arimatsu, S Kaewkes, T Laha, S J Hong, B Sripa. Rapid Detection of Opisthorchis Viverrini Copro-DNA Using Loop-Mediated Isothermal Amplification (LAMP). Parasitol Int 2012. [Google Scholar] [Crossref]
- N J Benedetti, T S Desser, R B Jeffrey. Imaging of Hepatic Infections. Ultrasound Q 2008. [Google Scholar] [Crossref]
- B I Choi, H J Kim, M C Han, Y S Do, M H Han, S H Lee. CT Findings of Clonorchiasis. Am J Roentgenol 1989. [Google Scholar]
- Y Y Jeong, H K Kang, J W Kim, W Yoon, T W Chung, S W Ko. MR Imaging Findings of Clonorchiasis. Korean J Radiol 2004. [Google Scholar] [Crossref]
- S Mas-Coma, M A Valero, M D Bargues. Chapter 2 Fasciola, Lymnaeids and Human Fascioliasis, with a Global Overview on Disease Transmission, Epidemiology, Evolutionary Genetics, Molecular Epidemiology and Control. Adv Parasitol 2009. [Google Scholar] [Crossref]
- M L W Spengler, H Isseroff. Fascioliasis: Bile Duct Collagen Induced by Proline from the Worm. J Parasitol 1983. [Google Scholar]
- A M El-Shazly, H A El-Nahas, M E Soliman, A A Abdel-Mageed, S El-Gharabawy, A T Morsy. Cholestasis in Human Fascioliasis in Dakahlia Governorate. J Egypt Soc Parasitol 2005. [Google Scholar]
- M A Valero, I Perez-Crespo, M V Periago, M Khoubbane. Mas-Coma, S. Fluke Egg Characteristics for the Diagnosis of Human and Animal Fascioliasis by Fasciola Hepatica and F. Gigantica. Acta Trop 2009. [Google Scholar] [Crossref]
- S Mas-Coma, M D Bargues, M A Valero. Diagnosis of Human Fascioliasis by Stool and Blood Techniques: Update for the Present Global Scenario. Parasitology 2014. [Google Scholar] [Crossref]
- P M Intapan, W Maleewong, C Wongkham, K Tomanakarn, K Ieamviteevanich, V Pipitgool, V Sukolapong. Excretory-Secretory Antigenic Components of Adult Fasciola Gigantica Recognized by. Southeast Asian J Trop Med Public Health 1998. [Google Scholar]
- Z A Demerdash, T M Diab, I R Aly, S H Mohamed, F S Mahmoud, M K Zoheiry. Diagnostic Efficacy of Monoclonal Antibody Based Sandwich Enzyme Linked Immunosorbent Assay (ELISA) for Detection of Fasciola Gigantica Excretory/Secretory Antigens in Both Serum and Stool. Parasit. Vectors 2011. [Google Scholar] [Crossref]
- B Sarkari, S A Khabisi. Immunodiagnosis of Human Fascioliasis: An Update of Concepts and Performances of the Serological Assays. J Clin Diagn Res 2017. [Google Scholar] [Crossref]
- A Kabaalioglu, K Ceken, E Alimoglu, R Saba, M Cubuk, G Arslan. Hepatobiliary Fascioliasis: Sonographic and CT Findings in 87 Patients during the Initial Phase and Long-Term Follow-Up. Am J Roentgenol 2007. [Google Scholar]
- W H El-Tantawy, H F Salem, N A Mohammed Safwat. Effect of Fascioliasis on the Pharmacokinetic Parameters of Triclabendazole in Human Subjects. Pharm World Sci 2007. [Google Scholar] [Crossref]
- H F Farag, A Salem, S A El-Hifni, M Kandil. Bithionol (Bitin) Treatment in Established Fascioliasis in Egyptians. J Trop Med Hyg 1988. [Google Scholar]
- A Olaya, M Ortiz, J Quispe Lazo. Fasciolasis in Cajamarca. Rev. Gastroenterol. Peru 2002. [Google Scholar]
- C K Parsak, I S Koltas, G Sakman, E U Erkocak, M Inal. Surgery in Fasciola Hepatica Pancreatitis: Report of a Case and Review of Literature. Z Gastroenterol 2007. [Google Scholar] [Crossref]
- M Echenique-Elizondo, J Amondarain, C Liron De Robles. Fascioliasis: An Exceptional Cause of Acute Pancreatitis. JOP 2005. [Google Scholar]
- Introduction
- Entamoeba Histolytica (Amebiasis)
- Pathogenesis of Entamoeba Histolytica
- Clinical Features of Amebiasis
- Diagnosis of Amebiasis
- Radiology
- Management of Amebiasis
- Role of Surgery in Amebiasis
- Colitis with hollow viscus perforation
- Abscess rupture into thoracic cavity
- Abscess rupture into peritoneum
- Ascaris Lumbricoides (Ascariasis)
- Clinical Features of Ascariasis
- Diagnosis of Ascariasis
- Radiological Features of Ascariasis
- Management of Ascariasis
- Role of Surgery in Ascariasis
- Clonorchis sinensis, opisthorchis viverrini & opisthorchis felineus
- Pathogenesis of cercariasis
- Clinical features of cercariasis
- Diagnosis of cercariasis
- Serology
- Polymerase Chain Reaction (PCR)
- Radiological features of cercariasis
- Management of cercariasis
- Fasciola hepatica & fasciola gigantica (Fascioliasis)
- The life cycle and pathogenesis of fascioliasis ([Figure 6])
- Clinical features of fascioliasis
- Diagnosis of fascioliasis
- Serology
- Conclusion
- Source of Funding
- Conflict of Interest
